Cell and Gene Therapy Manufacturing
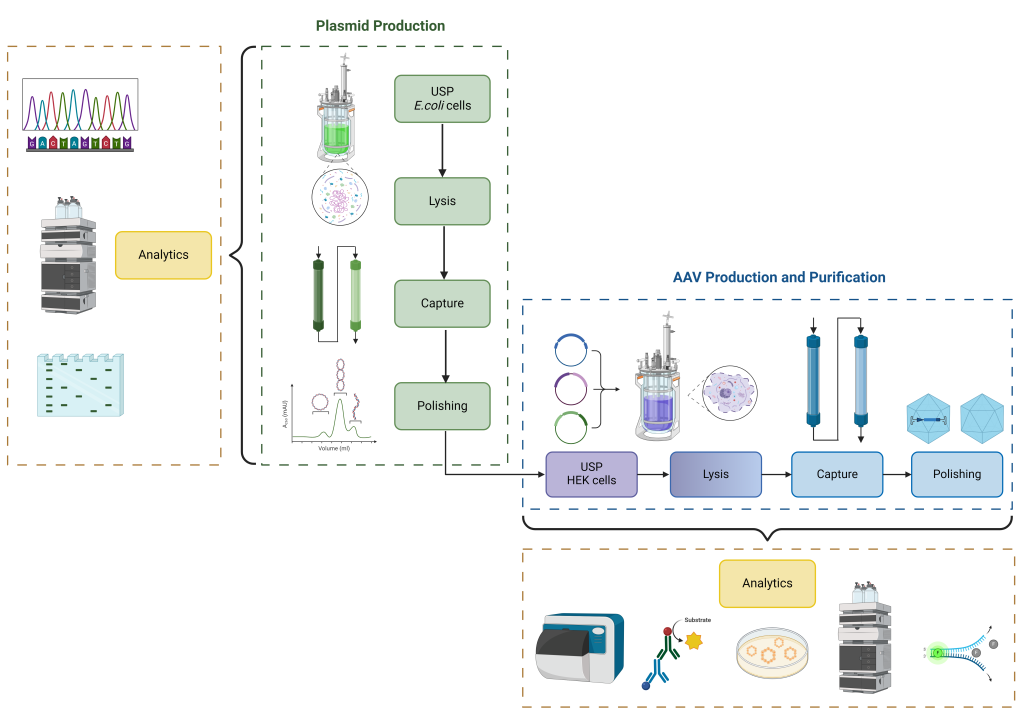
Adeno-associated viruses (AAVs) have great potential as delivery vehicles for gene therapy. The production of therapeutic AAVs is complex and requires many steps to get to the final product: Plasmid production and purification, cell expansion, plasmid transfection and finally AAV production and purification.
Here at FHNW, we cover all steps «from plasmid to product» for high-quality AAV production. We produce and purify plasmids with our in-house developed plasmid platform. We evaluate different conditions for plasmid transfection of mammalian cells to increase AAV yield in suspension, optimise production conditions in bioreactors and set up robust and reliable purification methods to later characterize the produced AAV particles.
Click on the items below for more information on the different production steps:
Motivated students have the chance to acquire essential skills in both upstream and downstream processing (USP and DSP) during internships such as microbial and mammalian cell expansion, bioreactor operation at small-scale (2L) up to larger scales (20-100L), plasmid transfection, viral vector production, setup of chromatographic techniques, Design of Experiments (DoE), data analysis using Python, etc. These techniques prepare students well for a smooth transition into various positions in the biopharmaceutical industry.