A New Tool for the Automated Sample Preparation of Whole Blood Samples by LC-MS using a Commercial Autosampler
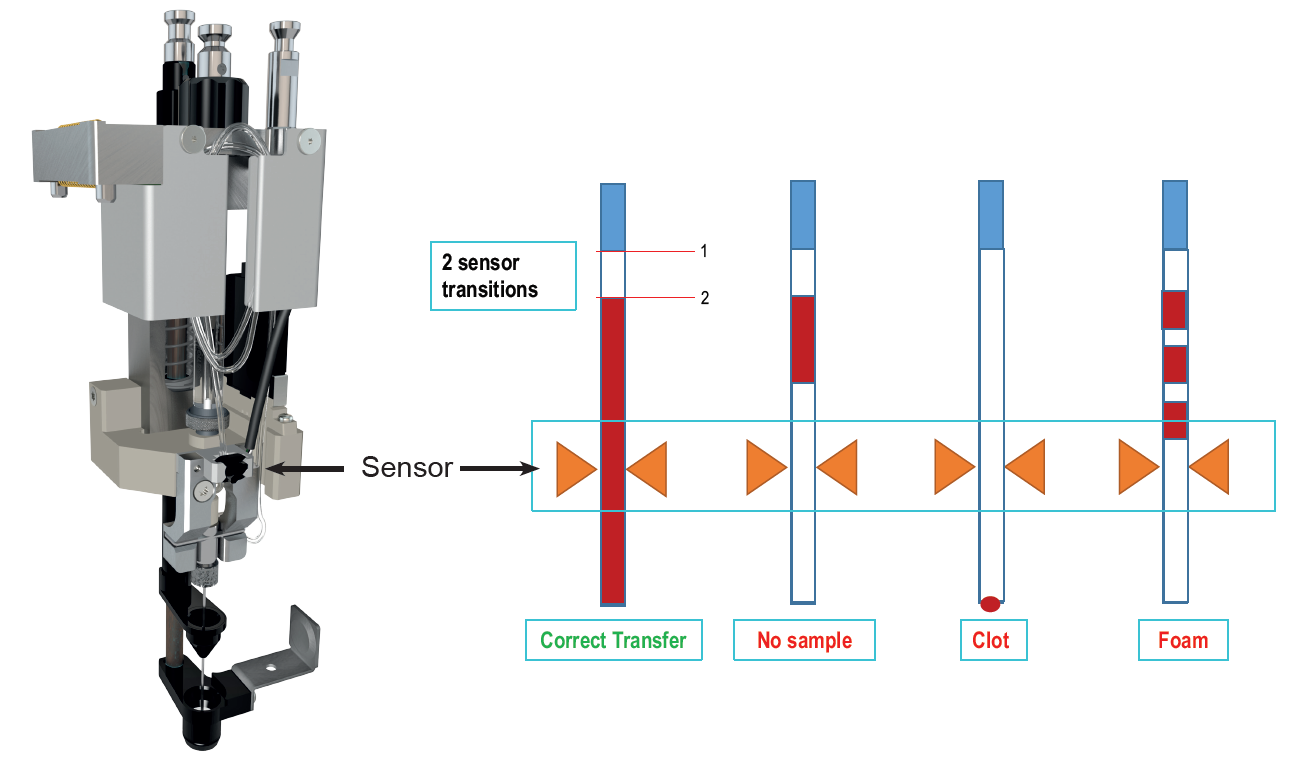
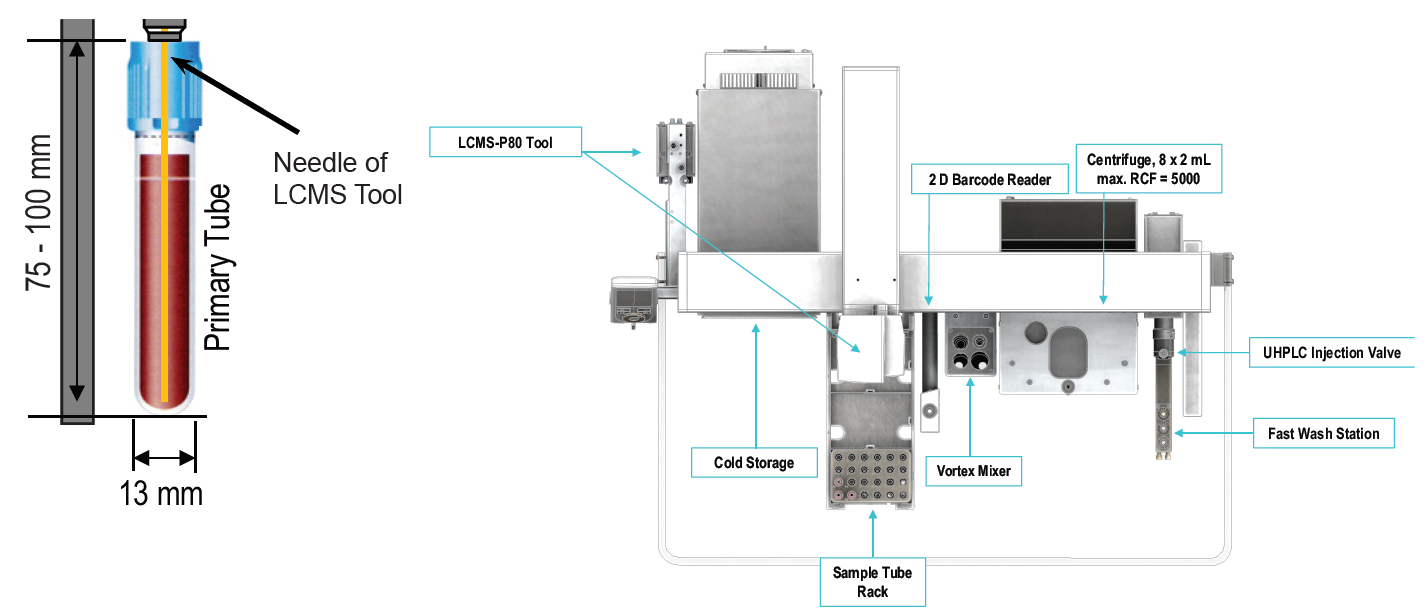
Automated sample preparation reduces cost per sample and minimizes sample handling errors. The use of robots is well established for therapeutic drug monitoring or diagnostics based on blood samples. Usually expensive and highly specialized pipetting robots are used. However, most of these systems are not designed with a direct interface for LC-MS applications and common pipetting systems are not optimized for smaller scale sample series. Here we present a new tool for liquid handling of whole blood samples and direct sample injection. The tool features an optical sensor that monitors all liquid handling steps. It detects presence or absence of sample, standards and reagents. The sensor is essential to ensure process safety for automated liquid handling steps.
Research by: Christian Berchtold[1],Günter Böhm[2], Renée Falsia[2], Thomas Preiswerk[2], Götz Schlotterbeck[1]
[1] University of Applied Sciences and Arts Northwestern Switzerland, School of Life Sciences, Institute of Chemistry and Bioanalytics, 4132 Muttenz, Switzerland
[2] CTC Analytics AG; Industriestrasse 20, 4222 Zwingen, Switzerland