“Less is more” – High-throughput nanoparticle detection for quality control of complex food matrices
Project in collaboration with A.Düsterloh & A.Otter, DSM Nutritional Products Kaiseraugst and Prof. C. Palivan, Department of Chemistry, University of Basel
Nanoparticles (<100nm) are wide-spread additives in various industrial products such as pharmaceutics, cosmetics, food products etc. as thickeners, taste enhancer, dye, appearance, thickeners, gelling and anticaking agents [1,2]. However recent studies concerning nanoparticles as trigger for diseases[3,4] e.g. including pulmonary inflammation, immune adjuvant effects and systemic effects including blood coagulation and cardiovascular effects[5,6,7] and the nanosize indeed enables to crossing natural body barriers such as the mucosa of the gastrointestinal tract or the blood brain barrier.[8] Nanoparticles are engineered in various sizes and shape and readily commercial available, thus the characterization of nanoparticles is a standard procedure and various methods such as DLS, UV/Vis, TEM, SEM give reasonable specifications of the pure particles. These methods are well established for high purity & particle concentrations, however recent interests in “nanoparticle”-free or -low products demand for methods that enable the detection of few nanoparticles and often in complex matrices, such as cremes, emulsions, suspensions etc. The matrices can contain additional particles, vesicles oils that can form micelles etc. which rises the level of difficulty for the distinct nanoparticle characterization tremendously. Therefore, standard protocols for the specification of nanoparticle free formulations is of high demand for the industry. The European Food Safety Authority currently proposed a “Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles”. [9] Moreover since May first, certain nanoparticles in foods have to be labeled with [Nano] in ingredients description. In future we expect such methods to be implemented in standard quality control of chemical processes, which demands for high throughput analysis and automated evaluation.
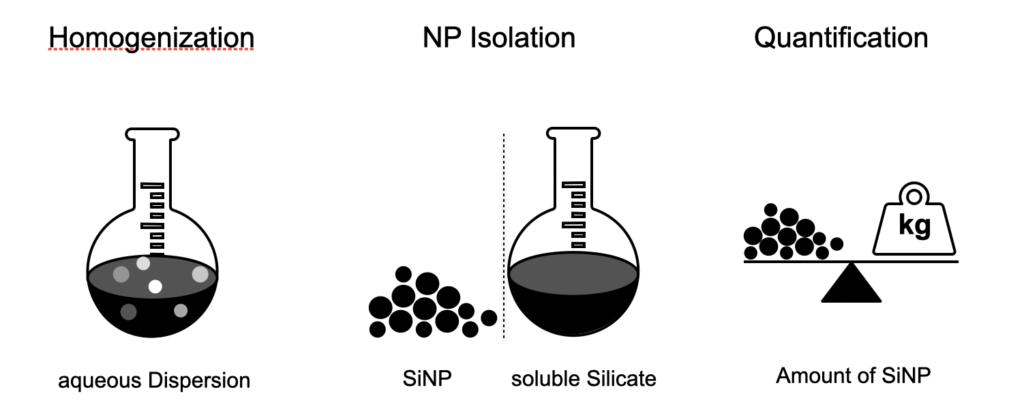
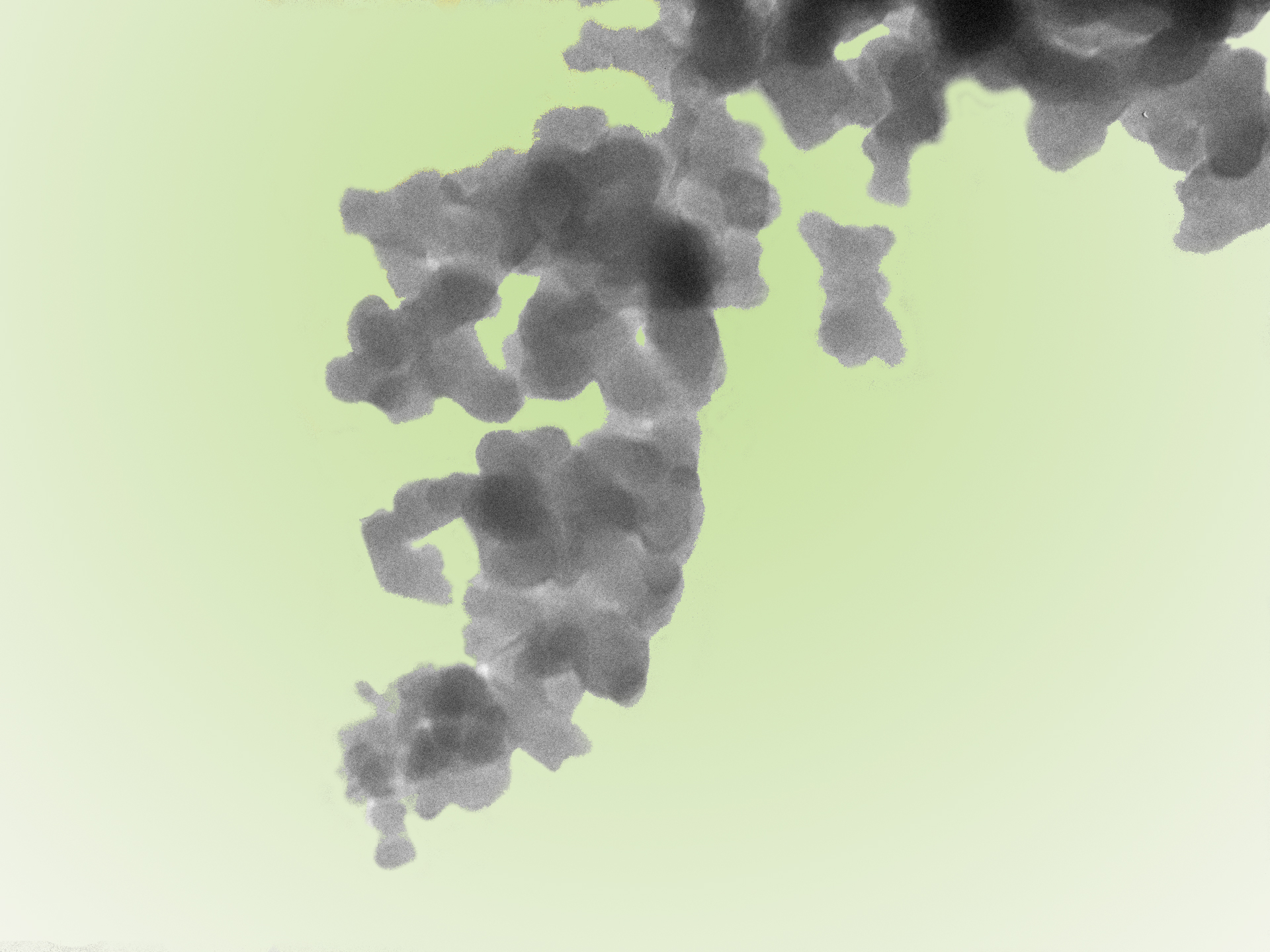
In this project we use silica nanoparticles (SiNPs), a common anticaking agent (AA) in processed food, as model system to establish characterization methods with different levels of accuracy and limits of detection versus speed, sample handing, and automatization. Sample preparation methods that enable to isolate SiNPs for a quantitative analysis are developed. Among the standard particle characterization techniques, we are also applying dynamic light scattering (DLS), energy-dispersive X-ray spectroscopy with a scanning transmission electron microscope (EDX-STEM), inductively coupled plasma mass spectrometry (ICP-MS) etc. in order to gain specificity and sampling speed. The final method aims to characterise food matrices of different production states and thus allow the quality control of the complete manufacturing process.
- Commission recommendation on the definition of nanomaterial , Official Journal of the European Union, L 275/38 (2011)
- M.Younes, P.Aggett, F. Aguilar, R.Crebelli, B.Dusemund, C.M.Filipi, M.J Frutos, P.Galtier, D.Gott, U.Gundert-Remy et al. Scientific Opinion on the re-evaluation of silicon dioxide (E 551) as a food additive. EFSA Journal, 16, 5088, 70 (2018)
- N. Prajitha, S.S. Athira, P.V. Mohanan, Bio-interactions and risks of engineered nanoparticles, Environ. Res, 172, 98-108, (2019),
- K.Donaldson,L.Tran, L.A.Jimenez, R.Duffin, D.E.Newby, N.Mills, W.MacNee, V.Stone, Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol., 2, 10 (2005).
- B.Granum, M.Løvik, The Effect of Particles on Allergic Immune Response, Toxicological Sciences, 65, 7-17, (2002),
- P.J.A.Borm, W.Kreyling, Toxicological hazards of inhaled nanoparticles-potential implications for drug delivery, J. Nanosci. Nanotechnol., 4, 521-31, (2004).
- G. Oberdörster, E. Oberdörster, J. Oberdörster, Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles, Environ Health Perspect., 113, 823–839 (2005)
- Simon More, Vasileios Bampidis, Diane Benford, Claude Bragard, Thorhallur Halldorsson, Antonio Hernández-Jerez, Susanne Hougaard Bennekou, Kostas Koutsoumanis, Kyriaki Machera, Hanspeter Naegeli, Søren Nielsen, Josef Schlatter, Dieter Schrenk, Vittorio Silano, Dominique Turck, Maged Younes; Jacqueline Castenmiller, Qasim Chaudhry, Francesco Cubadda, Roland Franz, David Gott, Jan Mast, Alicja Mortensen, Agnes G. Oomen, Stefan Weigel, Jose Tarazona and Reinhilde Schoonjans, “EFSA GUIDANCE ON TECHNICAL REQUIREMENTS FOR REGULATED FOOD AND FEED PRODUCT APPLICATIONS TO ESTABLISH THE PRESENCE OF SMALL PARTICLES INCLUDING NANOPARTICLES” Draft 9.July2020, https://www.efsa.europa.eu/en/consultations/call/public-consultation-draft-efsa-guidance-technical-requirements
Kontakt
Head of nanoLab
FHNW – School of Life Sciences
nanoLab
Hofackerstrasse 30
4132 Muttenz
Switzerland
Comments
No comment posted about “Less is more” – High-throughput nanoparticle detection for quality control of complex food matrices